| Lead Investigators | Dr. Roopinder Sandhu MD, MPH and Dr. Ross Tsuyuki BSc Pharm, PharmD,MSc |
| Study Sponsor | Heart and Stroke Foundation of Canada, Canadian Stroke Prevention Intervention Network (C-SPIN) & University of Alberta Hospital Foundation |
| Central Coordinating Centre | EPICORE/SPOR, Department of Medicine, University of Alberta |
| Background |
Atrial fibrillation (AF) is a leading cause of stroke, accounting for 15% of strokes overall and 25% of strokes in the elderly. The potential burden of AF associated stroke is already a critical health issue which will only increase since the prevalence of AF and stroke attributable to AF increases substantially with age; more than 25% of Canadians over the age of 75 will develop AF. Oral anticoagulation (OAC) is a well-established therapy for stroke prevention. Despite robust evidence OAC therapy is effective and safe; the availability of simple stroke risk stratification schemes and numerous guidelines providing strong recommendations regarding who would benefit from OAC therapy, less than two-thirds of eligible patients are prescribed OAC therapy. The public health consequence of undertreated AF are enormous, thus, alternative strategies to deliver stroke prevention therapy, such as pharmacist-directed interventions, need to be explored. |
| Study Hypothesis | Among participants 65 years who have ‘actionable’ AF, the rate of optimal oral anticoagulation (OAC) therapy (either new prescriptions or appropriate changes to existing prescriptions) will be higher in the intervention arm (pharmacist) compared to the control arm (usual care). |
| Study Objectives |
Primary Objective: Secondary Objectives: |
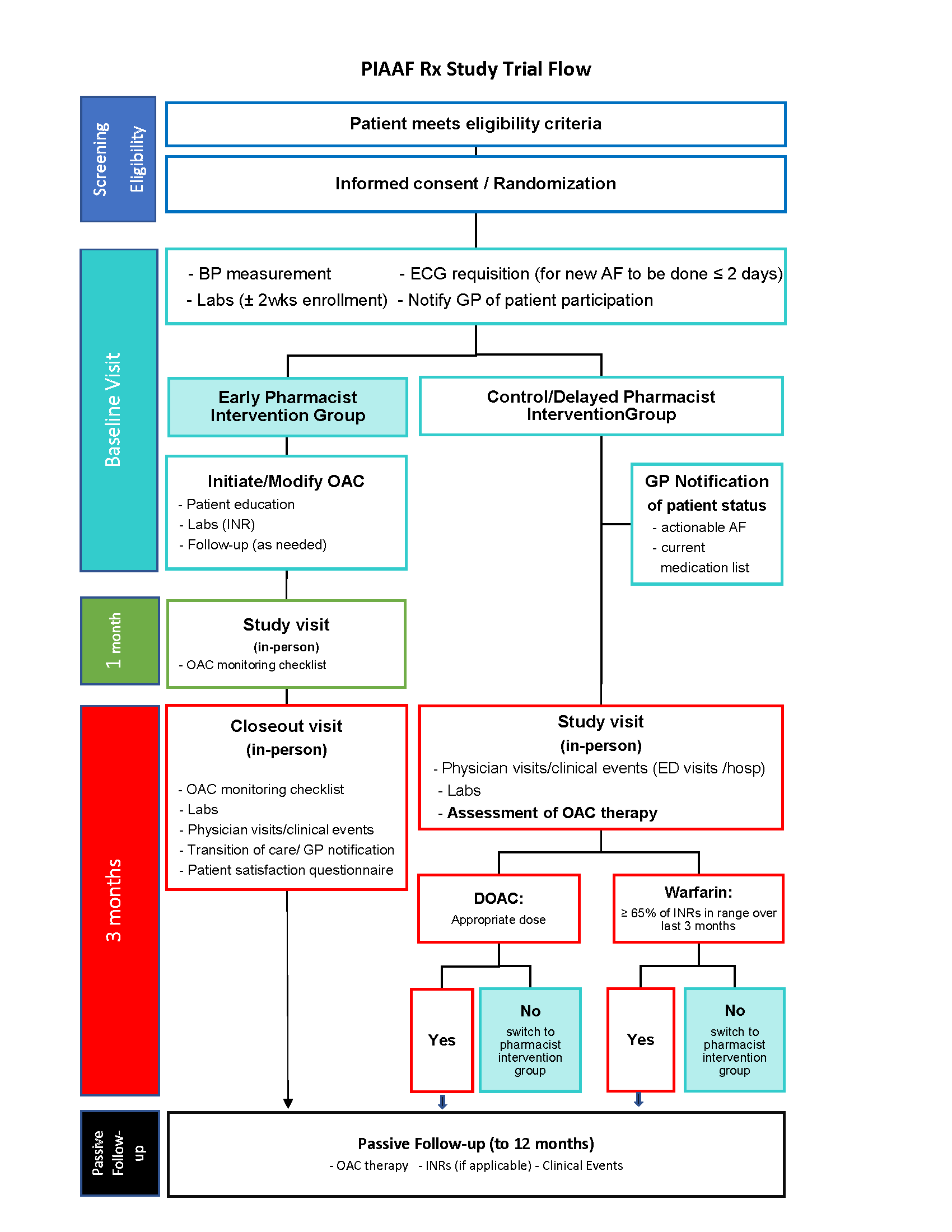
| Study Design | Open label randomized clinical study in participants 65 years or older with “actionable AF” randomized to pharmacist care versus enhanced usual care. Eligible and consenting participants will be enrolled at community pharmacies. |
| Participants/StudyDuration | Total of 380 participants will be randomized and followed for three months. Patients in the usual care arm will be given the pharmacists intervention if OAC therapy is not optimal at 3-months. Thereafter passive follow up by Research Project Office to 1 year. |
| Study Population | Participants 65 years or older with AF who are not on optimal OAC therapy. |
| Inclusion Criteria | Participants will be eligible for inclusion if they meet the following 1. Age > 65 years. 2. Unrecognized AF with one additional stroke risk factor (hypertension, diabetes, heart failure - history of or left ventricular ejection fraction <0.40), previous stroke or transient ischemic attack 3. Previously diagnosed AF not on OAC therapy but eligible 4. Previously diagnosed AF on suboptimal OAC therapy requiring adjustments. 5. Written informed consent |
| Exclusion Criteria | 1. Inability to read or understand English 2. Participants considered unreliable by the Investigator or designated pharmacy team member concerning the requirements of follow-up |
| Screening | 1. All patients age > 65 years with one additional stroke risk factor with unrecognized AF undergo AF screening using a 30 second single lead ECG recording device (AliveCorTM). The single lead ECG will be confirmed by a Cardiologist. 2. Those with known AF will have a brief medical history and medication review. |
| Enhance Usual Care | GP notification by fax of study participation with AF diagnosis and list of medications. |
| Intervention | 1. Education on AF 2. Prescribing new or adjustment of OAC therapy 3. Laboratory monitoring 4. Bleeding risk assessment |
| Enrollment and Follow-up | Eligible and consenting patients will be enrolled at community pharmacies. All patients will have follow up at 3 months (and 6 months, if crossed over from enhanced usual care to “delayed” pharmacist intervention arm).Thereafter passive follow up by Research Project Team at the U of A |
| Assessment of Outcome Events | Appropriate OAC therapy as per Canadian Cardiovascular Society (CCS) 2014 AF guidelines |